
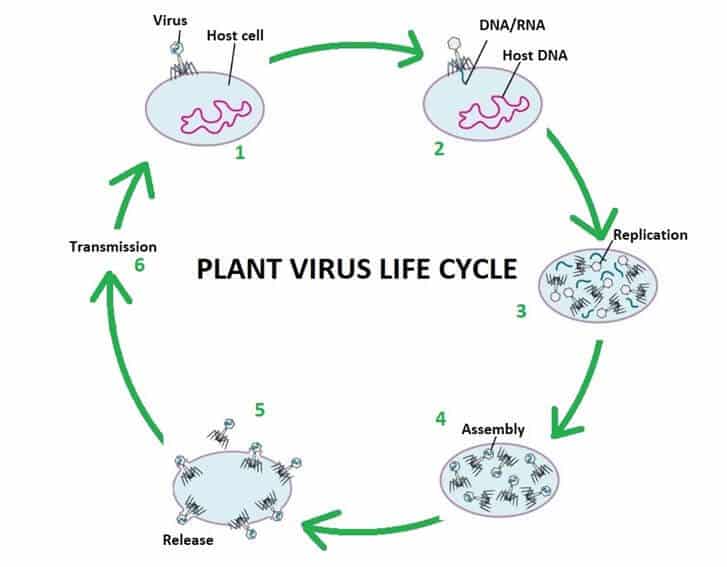
Plant viruses are viruses that affect plants. Like all other viruses, plant viruses are obligate intracellular parasites that do not have the molecular machinery to replicate without a host.
Plant viruses are pathogenic to higher plants. They can cause a dramatic decrease in yield, quality and shelf life and even plant death. Plant virus are made up of a strand of nucleic acid (DNA or RNA) surrounded by a protein sheath. Technically they are not living, as they are not cell based so cannot move or replicate by themselves. There is no cure for plant viruses, they must be managed with cultural practices and virus resistant plant stocks.

Plant viruses can cause major crop losses and greatly reduce quality and storage or products (vegetable/ornamentals and grains). Viruses can remain dormant and express when plants are unhealthy or stressed. They can join with other pathogens and plant viruses to form disease complexes that can decimate crops. They mutate very fast and new strains emerge all the time.
The symptoms of viral diseases in plants is important for virus identification and are often used to name the virus. The symptoms will change according to the plant virus strain / mixed virus infections, the host plant species, the nutritional status of the plant, the age of the plant, the stage of the infection and physiological growing conditions. Plant virus symptoms can be confused with bacterial and fungal diseases, nematode infections, plant nutrient deficiencies, abiotic stresses and herbicide injuries.
It is generally very difficult to identify a virus from symptoms alone. Viruses are sub-microscopic and plant samples should be sent to the laboratory for confirmation.
Viruses can be present but not expressed in healthy plants, weeds, and cuttings and seedlings. Symptoms can appear when the plants are stressed and in hotter weather. Multiple viruses can be present in one plant, and/or be present with bacterial or fungal infections that can form disease complexes that can be catastrophic with 100% plant loss.
Plant virus symptoms include, but are not limited to the following:-
By identifying the viruses infecting your plants you can look closely at the life cycle / the method of transmission/vector/host plants and level of damage that they can cause. Then you can make an informed, fact driven, scientifically based strategy to control it.
Propagation is a common form of virus transmission – it is important to screen plants, cuttings, seeds, starter material for viruses to ensure that the material is clean and virus free at the onset of any project, especially projects in new areas.
Potato seed can be screened for viruses and bacteria before planting – this allows the farmer to make an informed decision to use farm saved seed or buy in new seed. This also applies to other seeds for e.g. wheat, soybean & tomato seed. Sometimes, if you are aware you have a virus in your crop a special seed treatment can clean the seed.
Viruses are technically non-living and cannot move around by themselves. They require a transmission method and often a vector. By removing the vector or understand the transmission mechanism you can put hygiene protocols in place to control the spread of plant viruses.
Viruses can be transmitted in the plant sap via direct mechanical transmission. If your hands or tools get plant sap on and you then touch a clean plant, you can move the virus around. Some viruses do not last long outside a plant (semi-stable) while some viruses like Tobacco mosaic virus (TMV) and Tomato mosaic virus (ToMV) are very robust and stabile and remain infective for 2 years after drying.
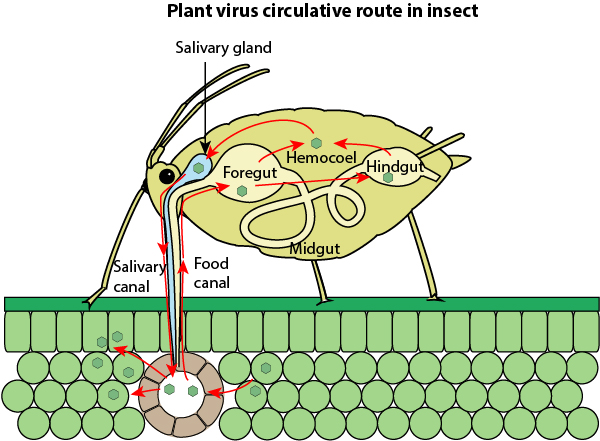
Plant virus vectors are generally insects that carry move the virus around on their mouth parts or salivary glands. The viruses can be transmitted by insects persistently – the insect acquires the inoculum and can transmit the virus indefinitely until the insect dies. Insects can transmit the virus non-persistently – the virus moves just to one plant, or semi-persistently – where the inoculum can remain infective for several hours.
Plant viruses generally cannot survive on their own and tend to live and perpetuate in host plants. These are neighbouring plants, commercial crops, volunteer plants or weeds that breed the virus up and act as reservoirs for new inoculum. Very often the host plants will show no symptoms. It is important to identify the potential reservoirs and remove the plants to stop the virus cycle continuing from one planting to another.
The following section contains information on viruses that we can screen for in our CropNuts Pathology laboratory. Viruses are tested using ELISA.
This is caused by the maize chlorotic mottle virus (MCMV) combining with a potyvirus, in Kenya this is the sugarcane mosaic virus (SCMV), to create a virus disease complex in the plants that is potentially fatal. The SCMV was first identified in Kenya in the 1930’s and is widespread, not causing much damage as our germplasm is quite resistant to it. MCMV was first noticed in Kenya in 2012 and caused up to 100% yield loss in the areas that were affected.
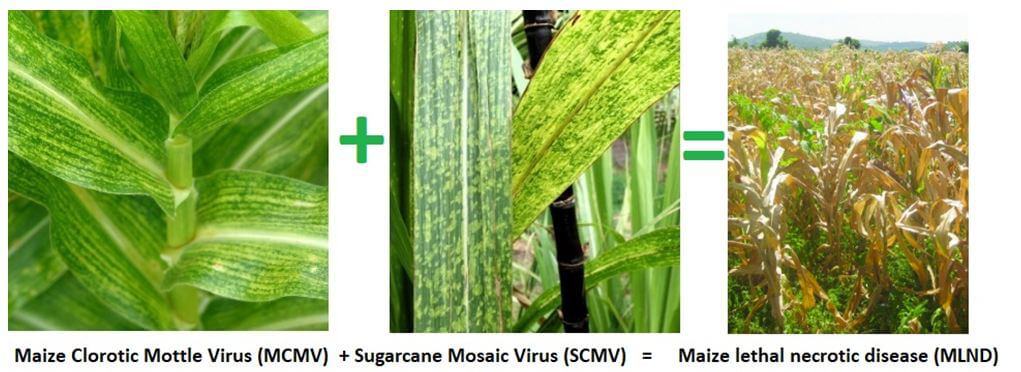
Transmission from place to place is via seed, in the seed coating, and inter-plant transfer is via several insect vectors. Testing seed plants, rogueing (removing and burning) symptomatic plants, special seed cleaning treatments and planting resistant varieties go a long way to reduce spread and damage.
This was the first plant virus to be discovered. It was isolated from Tobacco plants in the 1800’s. It has a wide host range of over 350 plant species. It can cause serious economic losses in tomatoes and peppers. The virus multiplies in living plant tissue but is very resilient and survives in a dormant state in dead plant tissue, retaining its infectiveness. It can also survive outside plant tissue in plant sap that has dried on tools and hands.
The most important method of transmission is mechanical transmission (direct, not via a vector or seed), on workers hands, tools, and clothing. It survives in dry sap, so if you touch an infected plant, then touch a door handle, the next person to touch the door handle can pick up the virus. Chewing insects (e.g. caterpillars and grasshoppers) can occasionally spread the virus but are not considered important in spread. Vegetative propagation perpetuates TMV and other viruses. TMV is found in all parts of infected plants. Infected stock plants should be discarded. Cuttings can be infected but show no symptoms. TMV can be moved around in tobacco products.
TMV Symptoms include stunting, mosaic patterns on leaves, malformation of leaves and growing points, yellow streaking or spotting. Like most viruses TMV cannot be diagnosed from symptoms alone. Plant material should be sent to our lab for confirmation.
ToMV is a closely related strain of TMV, it infects tomatoes most commonly, but can also cause problems in pepper, potatoes, apples and numerous weeds, including amaranth and lamb’s quarter.

The virus is initially seed-borne and can move large distances in seed. Only a few infected seedlings are required for the viruses to spread rapidly in a field. The viruses then spread from plant to plant via mechanical transmission. Both viruses are stable to drying so hygiene protocols should be particularly rigorous if these viruses have been found.
ToMV and TMV can exist for two years in dry soil, one month in moist soil and over 22 months in infected roots left in the soil. The viruses also stay infective in dried plant material and soil – and can be blown around by the wind. So, it is very important to remove plants, roots and all and burn and bury away from the field or greenhouse.
The viruses can persist in greenhouse structures for a long time.
There are no effective chemical controls against viruses.
Tomato Spotted Wilt Virus (TSWV) can cause serious crop losses in many economically important crops including vegetables, ornamentals and field crops. It has an unusually large host range (>35 plant families, >174 plant species including monocots and dicots). It is transmitted by thrips in a persistent manner. Many local weeds act as a reservoir.
Early accurate detection of the virus in infected plants and controlling thrips are critical measures in its control. The virus can be present on the seed coat, but not in the embryo. Seeds can be disinfected. However, seeds are not considered important for disease spread.
This table contains a partial list of common crops that can be affected by TSWV. The list continues to grow as new crops are identified or the virus mutates into new strains
| Field Crops | Tobacco, Peanut, cowpea, beans |
| Vegetable Crops | Tomato, pepper, potato, eggplant, lettuce, endive, celery, spinach, cucumber, cauliflower |
| Ornamentals / cut flowers | Chrysanthemum, Gypsophila, Aster, Snapdragon, Zinnia, Cyclamen, Begonia, Cosmos, Dahlia, Calendula, Primrose, Calla Lily, Gerbera, Amaryllis, Gladiolus, Peony, Delphinium, Ranunculi, Hydrangea, Geranium, Stock, Anemone, Salvia, Verbena, Phlox, Morning glory, Poppy, Ageratum, Impatiens, Coleus, Lobelia, Tiger Lily, Lupine, Marigold, Evening Primrose, nasturtium, Limonium |
Symptoms of TSWV can be very host specific. Common manifestations are:

Weed hosts are important reservoirs for TSWV and other viruses. TSWV has been picked up in the following weed families:-
This is most important in understanding how the virus spreads. It can be spread by all thrips species in a persistent way. The virus is acquired by the larval stage feeding on an infected plant. After a latent incubation period of 3-10 days, depending on the thrip species, the adult thrips become infective and can spread the TSWV virus from plant to plant for the remainder of their adult lives. They cannot pass the virus to their progeny.
A large pool of infected plants and overlapping thrips life cycles can lead to continuous virus spread here in Kenya. The thrips’ rapid development time (quicker in hot weather) and reproduction rate can allow an undetected infestation to become a major problem very quickly. Although not strong fliers, thrips can catch a lift on wind currents and clothing. Effective chemical control can be hindered by insecticide resistance build up.

Remove infected plants, control thrips, screen new plants, disinfect seed coatings, scout clean fields first and infected fields at the end of the day. Do not wear yellow or blue clothing. Protect greenhouses with thrip net. Screen in-coming plants for TSWV. Plant TSWV resistant varieties.
Infection in seedlings / young plants can cause the worst losses. Do not mix plant generations in one greenhouse. Do not mix ornamentals or infected cuttings in the same propagation house as seedlings/tissue culture plantlets. Rigorous control of thrips in propagation units is required (zero tolerance). Zero tolerance of weeds in greenhouses or fields. Screen mother plant stock and replace if infected.
Cucumber mosaic virus (CMV) is a Bromovirus with a worldwide distribution and very wide host range (>1200 plants – reputed to be the widest host range in the world). It is transmitted in seeds, parasitic weeds (like dodder) and is moved from plant to plant via aphids and mechanically (it is semi-stable). It can be harboured in many weeds. The transmission by aphids is non-persistent – they take about 5 minutes to acquire the virus and after about 2 minutes the inoculum load decrease and after 2 hours is finished.
While first discovered in cucumber is infects many vegetable and ornamental plants. (squash, peppers, beans, tomatoes, carrots, celery, spinach, egg plant and many ornamentals).
Symptoms include stunting, yellowing, leaf mottling, mosaics, shoe-stringing, leaf, flower and fruit distortion. It can cause up 20% loss in vegetable crops and 100% losses in ornamentals (by affected leaf and flower quality).
There are no chemical controls and treating aphids with insecticides does not kill the virus. Buy clean certified seed and plants. Screen and discard infected mother stock. Remove all weeds. Wash and disinfect hands and tools. Move from healthy crops to infected crops. Select CMV resistant varieties.
Other names include Alfalfa yellow spot, Lucerne mosaic virus, potato calico virus, tomato necrotic tip curl virus. AMV is an alfamovirus spread by aphids. The primary source of inoculum is infected seed or pollen. AMV can cause important yield losses, reduced cold / dry weather survival, and increased susceptibility to other pathogens. It can reduce the mineral content of fodder crops . AMV has a wide host range including some important crops (peas, lentils, potatoes, tomatoes, tobacco, wheat, lucerne).
Symptoms include wilting, stunting, dwarfing, mosaics, ringspots, mottles and necrosis, depending on the strain, crop, crop stage and environmental conditions. Signs of infection can persist or disappear. Potato leaves can display a very distinctive calico mottling in potatoes, and cause mis-shaped cracked tubers.

Planting infected seeds/tubers in a field followed by an aphid infestation causes high levels of infection. Plant clean seed / tubers. Control aphid populations. AMV persists in fields in weeds, infected seed pieces and volunteer plants.
The virus is transmitted by aphids in a non-persistent way. The aphid feeds on an infected plant, acquires the virus and spreads it to only a few plants before it is all deposited.
Do not plant susceptible crops within a mile of infected lucerne / clover / wheat fields. The highest risk of infection comes when these crops dry up and aphids move into new crops looking for moisture. Planting potatoes and Lucerne on one pivot is not recommended. Controlling aphids with insecticide does not control the virus.
Potato virus Y (PVY) is a potyvirus, there are three strains. (PVYO, PVYN, PVYNTN). It affects solanaceous crops (potato, tomato, pepper, tobacco, nightshade, many weeds). It can cause 50-80% losses in highly infected potato fields and reduce tuber quality and result in post-harvest tuber death.
Symptoms vary according to the strain. PVYO causes mosaic on the leaves. PVYN causes necrotic sports of the leaves and tubers. PVYNTN causes necrotic spots on the leaves and necrotic rings on the tubers, which extend into the flesh.
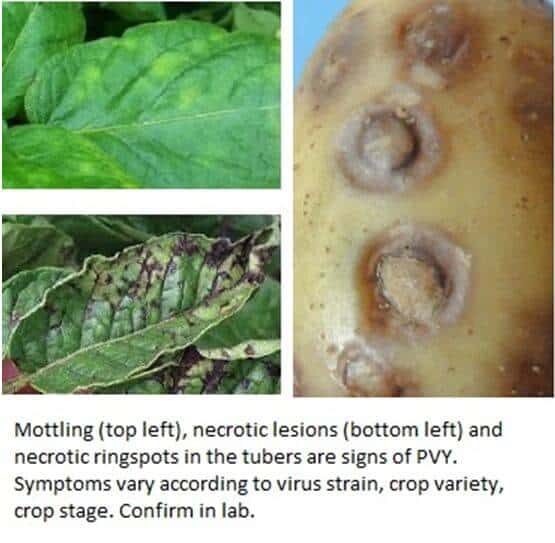
PVY is most frequently introduced into fields on infected tubers. It is then spread by aphids and farm equipment / tools / hands. The virus is transmitted by aphids in a non-persistent way, they can only transmit the virus to one plant. The virus persists in fields in tubers / volunteer plants / solanaceous weeds.
Remove weeds and volunteer plants. Insecticides have no effect on controlling the spread of the virus.
Citrus Tristeza (CTV) is a Closterovirus that affects citrus. Tristeza is means sad in Spanish. It has killed over 80 million trees worldwide. It is widespread in Kenya. Symptoms are highly variable and depend on the host tree / virulence and environmental conditions. The most common are quick and slow decline, seedling yellows, and stem pitting. Decline can be from several months to several years.

It is transmitted via rootstock and grafting material to fields, and by aphids in a semi-persistent manner inside nursery. Screen mother stock. Screen new orchards. Control aphids in young plants. Use CTV tolerant rootstocks.
If you’d want to test your plant samples, please send samples of your mother-stock for screening at our CropNuts pathology lab.Till next time,
Kindest regards,
Ruth

Ruth Vaughan is the Technical Director at Crop Nutrition Laboratory Services Ltd. (CROPNUTS). Ruth is also a contributing author to Kenya’s leading horticulture magazines such as the HortFresh Journal, HortiNews and Floriculture. Ruth is a great believer in soil health, organic matter, biochar and carbon sequestration as a way to alleviate climate change and increase food security. Loves visiting farmers and seeing all the different farming methods
Order our services and get to know how to improve your soil for better yeilds.