
Fall Armyworm control methods discussed in this article are tested by Cropnuts independent Agronomy team. The recommendations given in this article are independent and are not biased to any fall armyworm insecticide company in any way.
Fall Armyworm (FAW) or Spodoptera frugiperda , is a new emerging invasive pest that is wreaking havoc in Kenya and many other parts of the world, causing huge losses to farmers and impacting on food security.
Fall Armyworm is a caterpillar native to tropical and sub-tropical regions of the Americas. FAW was first detected in West and Central Africa early in 2016 and quickly spread across Sub-Saharan Africa. It got to Kenya early 2017. In July 2018 it was confirmed in India and early this year it reached parts of China. It has even been picked up as a quarantine pest on plant material entering the EU (exporters be aware).
The larvae of the fall armyworm feeds on many different crops, but particularly affects our maize, sorghum and sugar cane farmers, damaging the crop stand and substantially reducing yields. In the Americas, farmers have been dealing with FAW for centuries and much research has been done on this insect. We can learn a great deal from their previous knowledge.
In Kenya, as a new and emerging pest, we were caught unaware. Not only did we not know how to identify and deal with it, but, as a new species, the natural enemies of the FAW had not built up. So, the first outbreak of the FAW was disastrous. By understanding the nature and lifecycle of the pest, we can formulate better, more effective ways to deal with it.

Day 1-3 – the females lay egg clusters containing 100-200 dome shaped eggs underneath the leaves close to the stem junction at the base on the plant. If FAW populations are high they will lay eggs higher up the plant and on surrounding vegetation. The eggs are protected by a layer of scales between the eggs and over the egg mass.
3-5 days after the eggs are laid, the larvae emerge, begin feeding and migrate to the whorl (in maize). Young larvae can spin silken threads that catch the wind and transport them to new plants (ballooning, which can create 100% infestation in fields). The very young larvae only partially eat through the leaf creating transparent feeding windows in the leaves. They are greenish with a black head. As they get bigger, they can eat through the leaves, growing points and protective leaf bracts on the cobs, causing major destruction. Mature larvae are 3-4 cm long and vary in colour from light brown to green and black, they have a light-coloured inverted Y on their faces.
After 14-22 days the larvae mature and drop to the ground to pupate, forming a reddish-brown oval cocoon, 2-3 cm in length, that is hard to see. In soft soil they can burrow down 2-8 cm, in hard soil they can hide under leaf debris spinning a webbed cocoon.
8-9 days later the adult moths emerge. Their bodies are about 2.5 cm long, with a wingspan of 3-4 cm. They are mottled brown/ grey and difficult to spot. Adult moths are nocturnal and most active during warm humid evenings. The female starts laying eggs 3-4 days after emerging and can continue laying eggs for up to 21 days, although adult life duration is 10 days.
The length of the fall armyworm life cycle is affected by temperature, diet and humidity. Rising temperatures speed up the life cycle, cooler temperatures slow it down. Optimal temperatures are 28C for the larvae. In Kenya the life cycle is about 30 days, which means we can have 12 generations in a year! Frost kills the FAW, rain can wash the young larvae off the leaves and windy conditions will aid dispersal of the moths.

Maize is of course the crop most affected, but sorghum, millet, wheat and peas have also been under pressure.

Since 2017, I have undertaken several studies of the Fall Armyworm, including examining the effectiveness of different fall armyworm insecticides. One of the major challenges I found in this fall armyworm insecticide study was how to obtain reliable and meaningful results from a randomized, replicated field trial with an insect that is highly sporadic through the crop. This means that there is a danger that we confuse good control with plots or treatments where Fall Armyworm happen to be absent.
So, I went back a few steps and designed an experiment in which I collected larvae from the field, put them into batches according to size and placed them in identical containers with maize leaf and applied a known concentration of fall armyworm insecticide. This achieved the following:

All of the major approved fall armyworm insecticides were examined in a fully replicated (three times) with six larvae at 1-2 instar, six at 3-4 instar and six at 5-6 instar. The results after 5 days were as follows:
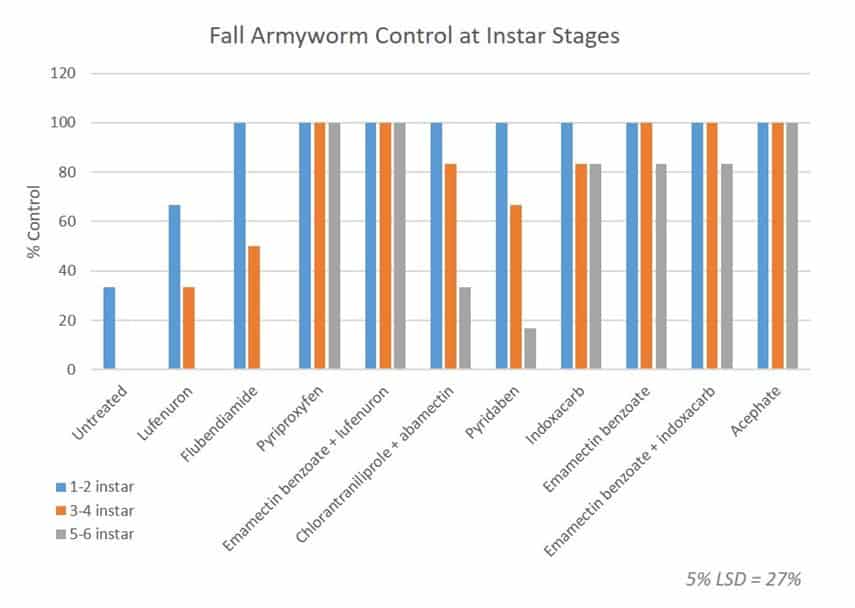
Note that the 5% Confidence Interval is 27%. So anything that has achieved between 73% and 100% control is not statistically different. In other words, pyriproxyfen, emamectin benzoate, indoxacarb and acephate containing products are all very effective.
Lufenuron performed poorly even on small larvae which is what our experience in the field suggests, while flubendiamide and chlorantraniliprole + abamectin showed poor performance on larger fall armyworm larvae. Nonetheless the later two are still important chemicals when applied at the correct stage, and bring a different mode of action to the control program so do not dismiss them.
Of course there are limitations. Some chemicals may be slightly more mobile in the leaf whorl because of locally systemic or vapour activity, so performance in the crop might vary. But the object of this study was to look at Fall Armyworm control as objectively as possible.
A few closing remarks:
My job as an independent agronomist is not to deliberately seek controversy, but to lay out the facts in an objective and scientific manner without fear or favour. I have NO relationship or commercial agreement with any manufacturers or suppliers of farm inputs, and doubtless the above results may not be well received by some individuals in the agricultural supply industry. Yet I feel that for the greater advancement of our knowledge as farmers and society it is important to research and to lay out our findings in an open and transparent way.
As a general rule, stick to manufacturers’ recommended dose rates. This is important not only to ensure good fall armyworm chemical control, but also to avoid and slow the development of resistance to the chemicals in the Fall Armyworm population.
The approved dose on the label should NEVER be exceeded; this is in place to protect the operator and the environment. Below are the typical amounts of active ingredient that I use for some common Fall Armyworm products:
Even though it is a new, emerging pest, fall armyworm has many natural enemies – predators, birds, bats, ants, earwigs, beetles, kill and feed off FAW. Parasitoids, tiny wasps that lay eggs in the eggs, larvae & pupae.
Pathogens infect and kill the fall armyworm include bacteria (BTs), viruses, fungi (Beauveria spp) and nematodes. Farmers would be well advised to be extra observant in the field so as to spot, conserve and promote the natural enemies. The cumulative effect of natural enemies can be very effective in FAW control and much longer lasting & more environmentally sound.
Plant early at recommended time taking advantage of the first effective rains. FAW populations build up during the season. Critical crop stages for maize are early vegetative growth and silking. Avoid late planting and choose a fast maturing variety to be ahead of the crowd.
Improved crop health will very much reduce the impact of FAW on yields. Good quality seed, appropriate plant spacing, good soil management and crop nutrition will boost vigour and reduce yield losses.
Hand picking and destroying the egg masses and larvae is very effective in small areas. Reducing immediate crop damage and appearance of 1500-2000 more larvae in a month! Chickens are great at finding the pupae and eating any larvae that fall off the plants. Don’t waste the larvae, an effective age-old natural remedy for caterpillar infestations is to collect the larvae, stress them for a few hours, and grind them up with water and spray on the crop, to promote the spread of natural pathogens.
Remove volunteer plants and infested crop residues. Eliminate grassy (and other) weeds in and around the crop, these can perpetuate high populations of FAW.
Intercropping with legumes will repel and confuse the adult female and deter her from laying eggs on the crop, AND benefit with nitrogen fixation to produce a more vigorous plant, as well as providing alternative food source for natural enemies. Crop rotation with non-grass crops such as cassava will reduce populations, but they can quickly build up.
Conservation agriculture: no till, reduced till, residue retention, crop rotation and cover cropping all increase biodiversity and numbers of natural enemies, as well as improving soil health, water infiltration and water storage of soils, to mitigate damage and yield losses.
Habitat management using a push plant that repels FAW e.g. Desmodium inside the maize plot, with a pull crop (Napier or Brachiaria) that attracts FAW on the outside, can be effective for small plots. Always remember to scout the pull crops to make sure they are not breeding up the pest!
Remember that the best way to deal with any new, emerging pests it to be prepared. Good soil health, increased biodiversity, promotion of natural enemies, Integrated Pest Management (IPM) and Good Agricultural Practices (GAP) are paramount in cushioning the negative effects of these outbreaks.
One of the most frequent calls that I hear is for Genetically Modified (GM) crops to be introduced to combat the pest and to reduce growing costs and risk of crop failure for farm of all sizes – small holder and large scale alike. Let us leave aside the ethical debate, but focus on the agronomic question of how effective this tool would potentially be. Would it put an end to our Fall Armyworm woes?
GM insect resistance – to Fall Armyworm, Corn Root Worm, Stalk Borer and Western Bean Cutworm amongst other species – is conferred by the presence of Bacillus thuringiensis (Bt) proteins in the plant which the larvae of the pest consumes and subsequently dies.
Many Bt variants have been developed over the last 20 years and bred into maize varieties in countries such as the United States and Brazil where the Fall Armyworm is prevalent, so we are able to observe their effectiveness over the years to understand how they might perform in conditions in East Africa.
The trend is frankly not encouraging. In recent years Brazil has seen around one third of the total maize crop planted in the traditional main season, and the remaining two thirds in the 2nd season. This has encouraged a continuous ‘green bridge’ of Fall Armyworm, similar to what we experience in Kenya, and has been attributed to increasing the selection pressure and development of resistance. In fact up to 8 generations of Fall Armyworm a year are recorded.
| Trait | Year Introduced | Year of first control failure |
| Cry1Ab | 2008 | 2011 |
| Cry1f | 2008 | 2011 |
| Cry1a.105 | 2009 | 2013 |
| Cry2Ab2 | 2009 | 2013 |
| Vip3a | 2009 | No resistance detected to date |
The major Bt traits effective on Fall Armyworm are listed above, and you can see that most have not endured under Brazilian conditions for more than a few years.
In the United States there is still partial control from some of these traits because of genetic variation in the populations, so it is important to understand that this does not mean that they are totally ineffective. Herculex based on Cry1f for example still provides acceptable control in some lower pressure regions.

However because of their cold winter, Fall Armyworm pressure is usually far lower until the pest moves North towards the major maize producing regions, by which time maize crops are generally larger and more advanced.
Vip3a or ‘Viptera’ is still effective but the pressure for resistance to develop is immense. Refuge areas of non-Bt maize are deliberately planted to allow Fall Armyworm that are NOT resistant to Bt maize to breed and disperse, providing genetic variation in the population. These would be essential if GM was introduced in Kenya, and would have to be well maintained by somebody.
Periodic spraying – probably in the early stages of the crop – would still be a requirement to reduce populations and the likelihood of resistance developing, and the cost of refuge areas would either need to be financed by government or the seed (trait) provider (which one would assume would have to be reflected in the cost of the seed).
So the message is that GM crops definitely do have a strong place agronomically and could reduce the amount of fall armyworm insecticides applied, but they are no silver bullet and managing the resistance threat would need to be well co-ordinated.
*This article is not an endorsement or criticism of GM crops, but an analysis of their likely effectiveness and how they would potentially fit into maize production in Kenya.
For more information on Fall army worm control or to join our agronomy newsletter list please contact us on support@cropnuts.com.

Farming for the future requires a change of approach. Monoculture, soil degradation and climate change and soil degradation are threats to the future of how we feed the planet. Agventure Ltd set up the Center of Excellence for Crop Rotation to help farmers diversify cropping systems and introduce techniques which have a long-term outlook to improve soil health. The Center of Excellence for Crop Rotation works extensively with Crop Nutrition Laboratory Services Ltd (Cropnuts).

David Jones is the Broad Acre Specialist at Crop Nutrition Laboratory Services Ltd. (CROPNUTS). David has a keen interest in soils and no till farming systems where he has undertaken work looking into weed levels and changes in soil structure, and has extensive experience in field trials and in the development of precision farming techniques. In his spare time he enjoys playing rugby.
Order our services and get to know how to improve your soil for better yeilds.