
In many places of the world, soil acidity is a substantial constraint to soil productivity. Sodium, aluminum, manganese, and hydrogen ion toxicity, as well as deficits in critical elements such as nitrogen, calcium, magnesium, phosphorus, and molybdenum, all contribute to plant growth suppression. Agricultural management practices have increased shifts in soil pH, raising concerns about soil acidity issues, particularly their effects on essential crop nutrients such as nitrogen (N) in Sub-Saharan African soils.
While a lot of approaches such as nutrient omission trials have been shown to be beneficial in adjusting surface soil acidity and improving total soil nitrogen in many farms, there are still severe soil nitrogen deficiencies. For instance, in the densely populated and heavily cropped counties of Kisii in southwestern Kenya, aggregate soil nitrogen losses through volatilization was estimated at 112 kg N, /ha/year, with serious P deficiencies. The situation is worse in the semiarid and subhumid areas which are dominated by sodic soils, an estimated 97 % of N applied inorganic N is volatilised due to higher soil pH, resulting to inherently low cereal yields and farming becoming a poverty trap in these areas.
More evidence on the impact of soil acidity on the soil’s nitrogen-supplying power (Figure 1), as well as other essential nutrient uptake and utilization by plants, is needed. We need to learn more about how soil affects plant-microbial interactions and microbes that transfer nutrients such as nitrogen around in order to figure out how it affects soil productivity in totality.
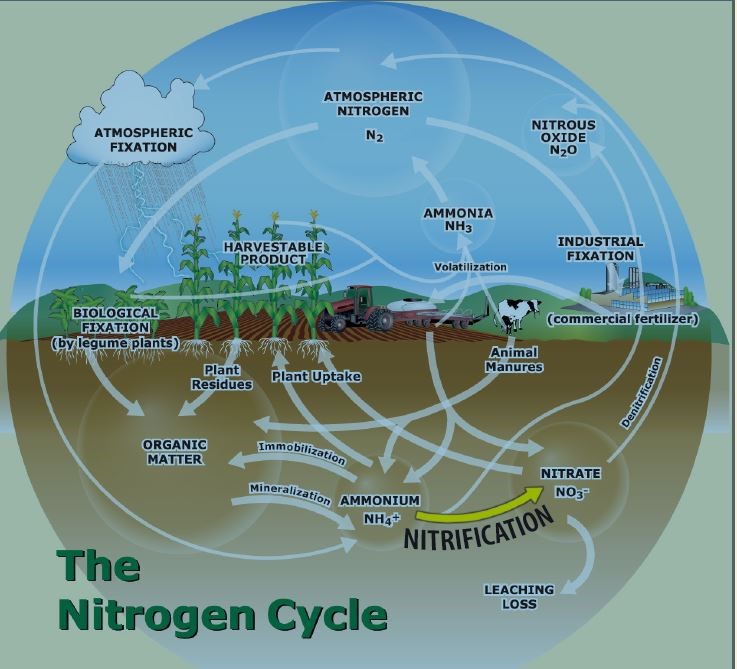
Figure 1: N supplying power pathways (adapted from www.ipni.net)
As minerals dissolve and organisms go about their business of digesting organic matter or fixing nitrogen, acids and bases are released. Acids and bases are discharged by plant roots, whereas acids are formed in the soil as a result of nitrogen fertilizer use. It is optimal for plants if the soil acidity level, also known as pH, does not fluctuate too much over the season. The pH scale is a technique for representing the quantity of free hydrogen (H+) in soil water, but it’s also linked to plant nutrient availability and the toxicity of specific elements like aluminum in soils. Because pH is measured on a log scale, soil with a pH of 4 is extremely acidic, and its solution is 10 times more acidic than soil with a pH of 5. Soil with a pH of 7 is neutral, meaning that the amount of base in the water is equal to the amount of acid. Although there are acid-loving crops, most crops thrive in slightly acidic soil with a pH of 6 to 7. Plants have more access to nitrogen in this pH range than in soils that are either more acidic or more basic.
Integrated soil acid management (ISAM) involving the combined use of animal wastes, composts, decomposing crop residues, decaying cover crops, or coating fertilizers containing urea with local knowledge has proven to be a cheap and quick way of managing soil nitrogen for optimum crop yield. Since the nitrification process occurs in the soil at a wide pH range, the ideal pH is thought to be between 6.5 and 8.8. In acid soils, nitrification is slower, and adding limestone to alleviate soil acidity often results in faster nitrification.
Nitrobacter activity can be inhibited at high pH settings (> 8.8). This situation allows ammonium to be converted to nitrite, but not the second step of nitrite to nitrate conversion. This could result in an undesired buildup of nitrite (unstable N form) in the soil linked to crop poisoning and loss of N in form of gas. Although nitrification occurs swiftly in most circumstances, ammonium does not accumulate to dangerous levels during the growing season. Localized high ammonium concentrations can arise due to the nature of fertilizer application and the tendency of ammonium to remain near to the place of application.
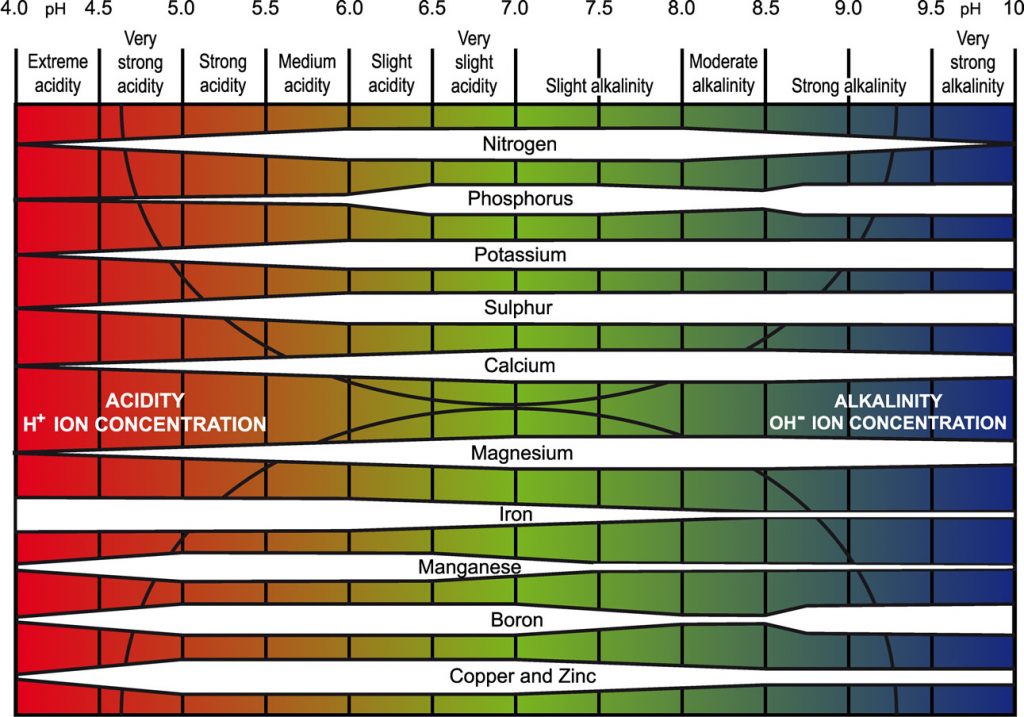
Influence of soil pH on plant nutrient availability. (adapted from pda.org.uk)
Following numerous applications of inorganic N-based residues that elevate ammonium concentrations and frequently cause shifts in soil pH. The application of fresh organic materials offers favorable conditions that are suitable for soil bacteria to convert it to preferred nitrate form via biological process, regardless of the source. Although there are differences in nitrification rates among various manures and composts (due to application rates, ammonium concentration, C:N ratio, etc.), they are rarely significant in field circumstances. Extremely high ammonia concentrations (such as those seen around a concentrated band of anhydrous ammonia) can momentarily stop nitrification. This is owing to the harmful effects of ammonia gas on soil bacteria, as well as the increased pH that occurs when concentrated bands of ammonia are present.
Although inorganic-based nutrients are concentrated, respective nutrients are readily available and therefore required at low rates. The source of ammonium in these conventional N fertilizers might affect the rate of nitrification, based on the following order: urea > diammonium phosphate > ammonium sulphate > ammonium nitrate > monoammonium phosphate. Therefore, an increase in acidity has been linked to some of the variations (for example, the pH of a DAP solution is approximately 8.0, while MAP is pH 3.5). These changes could be substantial for plant nutrition considering fertilizer N sources such as DAP and urea are separately used in large amounts by African smallholder farmers during planting and top dressing respectively.
Fertilizers containing urea should be used with caution. Nitrogen losses due to volatilization may occur if urea is not applied correctly, and urea may cause damage to developing seeds in some situations. Use irrigation or rainfall to quickly incorporate the urea fertilizer into the soil once it has been applied. When urea fertilizers are applied to the soil surface without being incorporated into the soil, more nitrogen is lost.
When using urea fertilizers, make sure the temperature isn’t too hot or too cold. Temperatures between 15- 20°C (70°F) in the soil are deemed sufficient. Using urea fertilizers with urease inhibitors – urease inhibitors lower the rate of hydrolysis and, as a result, the formation of ammonia and its volatilization. This gives you an extra one or two weeks to incorporate the urea.
In soils with a high pH, nitrogen losses through gaseous exchange are larger and efforts in using a variety of coatings to counteract the losses exist. For instance, regulating the pace of nitrogen losses through gaseous release has a number of advantages in terms of the environment, economy, and yield. Coatings on soluble fertilizers have been made from a variety of materials. Coatings are most typically applied to granular or prilled nitrogen (N) fertilizer, although they are sometimes applied to multi-nutrient fertilizers. Urea is the basis material for most coated fertilizers since it has the most nitrogen concentration (46 %), besides requiring microbial breakdown process.
The first extensively used fertilizer coating material was elemental Sulphur (S). It entailed spraying molten S over urea granules, then applying sealant wax to fill in any gaps or defects in the covering. Later, when the S layer was covered with a thin layer of organic polymer, the procedure was improved. Other coated fertilizers are created by reacting various resin-based polymers on the fertilizer granule’s surface. Another method involves combining low-permeability polyethylene polymers with high-permeability coatings. Manufacturers use different coating materials and techniques. To manage the nutrient release rate for individual applications, the fertilizer coating’s content and thickness are carefully modified. The time it takes for specific fertilizers to release nutrients might range from a few weeks to many months, depending on the product label.
Coating a fertilizer particle incurs an additional cost, hence coated fertilizers are more expensive than uncoated fertilizers. Coated fertilizers are effective in a wide range of agricultural and horticulture applications. They give a steady supply of nutrients that may have a variety of advantages:
Multiple environmental factors govern the pattern of nutrient release from coated fertilizers under a wide range of soil and cropping situations, making it difficult to predict. Many coated fertilizers, for example, release more quickly as moisture and soil temperature rise. Some products rely on microbial activity in the soil to release nutrients. Understanding the mechanics of nutrient release can assist producers in getting the most out of coated fertilizers. For instance, some coating materials are brittle, they are prone to abrasion and breaking in severe conditions, hence handling with care may result to desired results.
Apparently, the Integrated Soil Acid Management (ISAM) approach could be the best option for managing soil nitrogen, which is one of the most expensive and widely quoted as limiting soil productivity in African soil. So, we at CROPNUTS are at the forefront of designing farm-specific Integrated Soil Acid Management (ISAM) packages so that we can grow more with less money.
Kindest regards,
Stephen Wanjala Wamalwa
Technical Advisory Services
Cropnuts
Mr. Stephen W. Wamalwa has over 14 years of experience in multiple agricultural fields ranging from crop nutrition, fertilisers, and smart climate agricultural technologies. Mr. Wamalwa holds a Master of Science in integrated soil fertility management, a Bachelor of Science in agriculture, and a number of professional certificates. He is also skilled in GIS, crop nutrition report interpretation, the Statistical Package for Social Sciences (SPSS), Genstat, newsletters, and scientific report writing.
Order our services and get to know how to improve your soil for better yeilds.