
Soil pH is a critical factor that influences the availability of nutrients to plants. Understanding the relationship between soil pH and nutrient availability is essential for effective fertilization. Here are the key points to consider when discussing soil pH and its impact on nutrient availability:
Soil pH is a measure of the soil’s acidity or alkalinity. It is measured on a scale from 0 to 14, with 7 being neutral. Values below 7 are acidic, and values above 7 are alkaline.
Soil pH affects the solubility of nutrients. Some nutrients are more available to plants in acidic soils, while others are more available in alkaline soils.
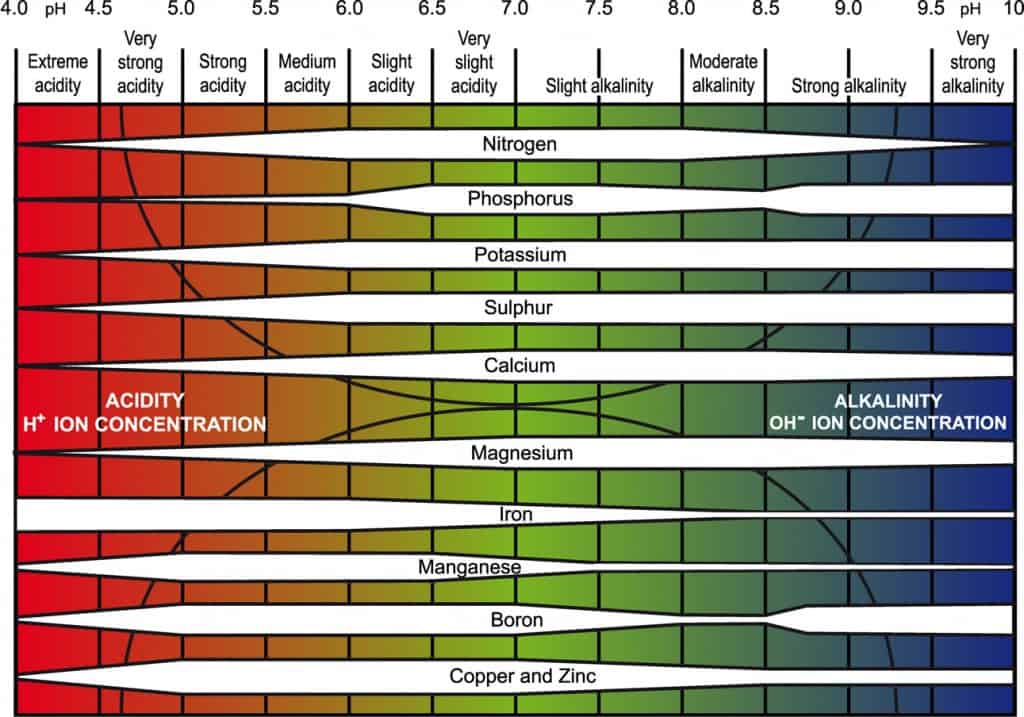
Nitrogen (N), phosphorus (P), and potassium (K) availability is influenced by soil pH. Nitrogen is more readily available in slightly acidic to neutral soils. Phosphorus availability is optimal in slightly acidic soils, and it decreases in strongly acidic or alkaline conditions. Potassium availability remains relatively consistent across a broad pH range.
Micronutrients, such as iron, zinc, and manganese, are especially sensitive to pH levels. They often become less available in alkaline soils. Iron chlorosis, a condition where leaves turn yellow due to iron deficiency, is a common issue in high-pH soils.
Farmers can adjust soil pH through liming (raising pH) or acidification (lowering pH). These practices are used to create conditions that favor nutrient availability.
Liming materials, like agricultural lime, are commonly used to raise pH in acidic soils. This enhances the availability of certain nutrients. Lime can also supply calcium, which is essential for crop root and cell development.
In some cases, acidification is necessary to lower pH, especially in soils where nutrient imbalances exist due to high alkalinity. Acidification can be achieved through the use of elemental sulfur or other acidifying materials.
Different crops have varying pH preferences. For example, tea crops thrive in acidic soils, while asparagus prefers neutral to slightly alkaline conditions.
Regular soil testing is crucial to monitor pH levels and ensure they are within the ideal range for the crops being grown. Maintaining the right pH level ensures that crops can access the nutrients they need for robust growth, reducing the risk of nutrient deficiencies or toxicities.
In conclusion, understanding the relationship between soil pH and nutrient availability is crucial for informed and effective soil management.
Farmers and agronomists can optimize crop health and yield by regularly testing their soil, and monitoring & adjusting soil pH as needed to meet the specific requirements of the crops they cultivate.
For personalized assistance in managing soil pH and nutrient availability, please reach out to our experts at support@cropnuts.com.
Grow more with less
#savesoil #soilhealth #soilscience
Order our services and get to know how to improve your soil for better yeilds.