
Calcium Nitrate is an inorganic compound with the formula Ca(NO3)2 and is mainly used as a component in fertilizers.
In soil and plant nutrition Calcium is King. Calcium Nitrate is the only cheapish non-reactive water soluble calcium fertilizer suitable for drip feeding fertigated crops, so it goes without saying that calcium nitrate is the sovereign of fertilizers. There are NO realistic, long term alternatives. Sadly, Kenyan (and Ugandan) growers have encountered a glitch in Calcium Nitrate supply due to port delays (October 2018). A long delay in clearing containers (and the extra costs) have resulted in lower availability and higher prices.
What can Kenyan growers do? As already mentioned, there are no direct alternatives to Calcium Nitrate, so we are going to have to be brave, innovative and move into untested waters…….
Calcium is not called the King of nutrients for nothing. Calcium is very important for soil health, microbial diversity, soil structure, heavy metal protection and countless other soil properties. As a soil amendment, we do have alternatives. Soil growers that are following our soil health program and balancing calcium and pH levels at planting, and annually, should have adequate Calcium levels in their soils, which will hold them in good stead through this crisis. They will be able to “mine” their soil Calcium and top it up with topdressing of Gypsum, Calcitic lime or dolomitic lime (depending on the soil test result recommendations).
Hydroponic growers are no so lucky. At planting growers should condition their media with gypsum, lime, calmag to bring up the calcium levels to optimum at the start – but the amount the media can store is very limited and the calcium very quickly (in a matter of days!) runs out. The whole concept behind hydroponics is to be able to have a system of nutrient feeding that can be changed at the drop of a hat…
Calcium is an essential secondary plant nutrient, and typically supplied in nutrient solutions at a rate of 80-240 ppm. Crucial for plants, Calcium also prevents various other nutrient deficiencies and is an essential ingredient of strong cell walls which leads to sturdier crops with increased vitality and storage properties (critical for exported crops that need to reach their destination in good form!).
Calcium is deposited as calcium pectate in the cell walls. This means it is not mobile in the plants and cannot be moved from older tissue to younger tissue, it is therefore needed all the time. Important for all plants – Calcium is especially important in roses, tomatoes, peppers, brassicas and cuttings.
Calcium enables the uptake of other nutrients. It is important for root cell division and cell strength. Low levels of calcium in root tissue make the roots susceptible to soil borne diseases and nematodes. Without root division phosphorous uptake slows down.
Calcium is important for the stability and functioning of cell membranes. When Calcium supply slows down the cell membranes become leaky, and cell division is disrupted – causing twisting and cupping of the newer foliage. Leaky membranes are more susceptible to pre-harvest diseases and post-harvest botrytis and bruising.
Calcium slows the ageing of cells by protecting them from toxins and ethylene – hence its importance in maintaining quality and shelf life. For cuttings producers – ample calcium levels are vital -not just for shipping, but also in root cell formation for rooting.

Because it is immobile, calcium deficiency occurs in the newest growth, and symptoms include tip burn in leafy crops, and blossom end rot of tomatoes and capsicum. Softer ‘leaky’ cell membranes are more susceptible to spray damage and heat stress.
Calcium deficiency symptoms vary according to plant type, variety, degree of severity of the calcium deficiency and growing conditions. At the onset symptoms are difficult to spot, and it is important in this difficult time, to monitor calcium levels with regular leaf analysis.
By the time Calcium deficiency expresses symptoms, cupping of new foliage, pale marginal bands, increased spray damage, diseases and insect pressure, water-soaked areas on stems and leaves and root tips that become jelly like, yield and quality will have been affected beyond repair.
Keep a close eye on your flower and vegetable shelf-lives. It’s not just the cells on the outside that are affected, Calcium deficiencies affect the inside cells too. Look out for black heart in lettuces, empty cabbages, browning inside of flower buds, browning in cut beans, pithy baby corn.
Optimum levels of Calcium in leaves vary from plant to plant, but typically fall in the range of 0.8-2%. The age of the leaves for sampling is important – because it is immobile older leaves will generally not show a deficiency. Hence the need for sampling the youngest most mature leaves for leaf analysis – in roses this would be the 3-4 leaf down on roses showing color. Calcium deficiency can cause loses of > 50%! So, it is important to stay on the ball.
Optimizing Calcium nutrition in a crop is not just a matter of dosing correct levels. Calcium uptake is complex and often misunderstood. Soils, media and nutrient solutions generally have enough Calcium levels and many Calcium deficiencies are induced deficiencies.
Environmental factors, crop factors, solution management, moisture levels and elemental ratios all interact to affect Calcium uptake. Understanding the factors that affect Calcium uptake will help us maximize the limited calcium we have and will influence our choice of replacement fertilizers.
Calcium uptake is a passive process and Calcium moves into the root and through the plant in water movement driven by the transpiration stream through the xylem. So, it goes without saying that anything that factors that influence transpiration will affect the uptake of calcium.

Warm, humid conditions combined with limited airflow and rapid growth are known as ‘calcium stress periods’ and result in tip burn & death of growing points (blind shoots).
Plants take up less water, and therefore Calcium from dry root zones. EC builds up around dry roots creating an osmotic stress, reducing water and calcium uptake. Flooded roots have no oxygen and shut down = no calcium uptake. Optimum moisture = optimum root activity = optimum Calcium uptake. If you haven’t got one, get a moisture sensor like Aquacheck, and use it to guide you on your irrigation cycling.
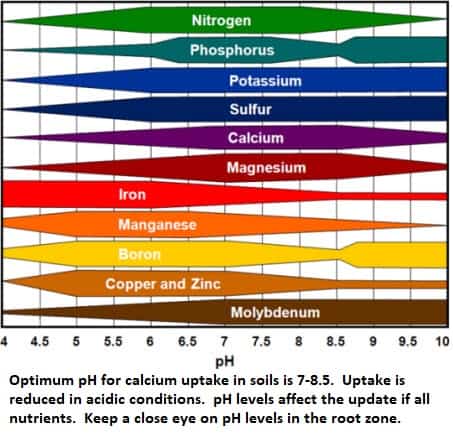
pH has a big effect on Calcium availability and uptake. Low pH increases the solubility of metallic cations which depress Calcium uptake. At high pH calcium is bound up as an insoluble carbonate (Lime), and acidic conditions are required to neutralize the carbonate and release the Calcium.
When planting in the soil do a complete soil analysis at the the start. Scientifically calculated additions of lime / dolomitic lime, gypsum or sulphur will help balance your soil pH and optimize Calcium levels. For media, analysis and pre-conditioning of media will balance media pH. Cocopeat and pumice can contain large amounts of Sodium and Potassium and absorb large amounts of Calcium and Nitrogen. Conditioning needs to be done pre-planting.
The irrigation water quality, amount of acid and ratio of nutrients all affect the immediate and long-term pH in the root zone.
At low ECs plants struggle to take up any nutrients. High ECs in the root zone cause osmotic stress, reducing water and Calcium uptake. Take extra care in maintaining the optimum EC in the root zone. Note that drip & drain analysis in hydroponics can be misleading with regards to EC levels in media, a phenomenon known as false drain.
Monitor EC in the root zone with regular sampling for a 1:1.5 media analysis, or a 1:2 soil analysis. Samples should be taken fresh, not dried out, and shipped to the lab as soon as possible and not over the weekend. Analysis is time and moisture sensitive. Sending samples in absorbent bags (eg paper) will suck out the moisture and give a false high reading.
EC in the root zone is a balance between EC soil/media, of drip and the water uptake of the plants.
Most well-balanced nutrient solutions at the correct EC range contain enough calcium for optimum uptake. Balance is the key here.
The health of the root system plays a vital role in calcium uptake. Root diseases, nematodes, water logged roots, anaerobic root zones all restrict calcium uptake. Run regular nematode and root borne disease checks.
Next, we will discuss calcium nitrate alternatives.
As mentioned earlier, there are no water soluble, non-reactive Calcium Nitrate alternatives that you can throw into your fertigation recipes as a direct replacement. So here I will list some of the options available for Calcium to boost Calcium levels.
NOTE FIRSTLY that Calcium Nitrate brings a huge contribution of nitrates into your fertigation recipe. This is a major essential nutrient and the first thing any grower should do when removing calcium nitrate from their recipe is ensure that the N level is topped up via Ammonium sulphate, MAP, potassium nitrate and magnesium nitrate sources. Nitric acid is also a very good source of N, when balanced with lime or bicarbonates.
Without the Calcium in soluble form the EC in the recipe can come down – it will be balanced by the topdressing. When balancing the Nitrogen – bear in mind that ammonium can be very acidifying AND is a cation – so competes with Calcium uptake. Ideally you need to apply the Nitrogen in nitrate form.
There are two types of lime.
Lime comes in dust form and can be hard to measure and messy to apply. It is generally mined in Kenya and therefore cheaper and more easily available than imported limes. The Calcium Carbonate Equivalent (CCE) of a good lime should be >70%. It is determined by quality and the fineness of the lime. The finer the lime, the faster it will react with the soil – releasing Calcium and taking the pH up. Large lumps are every unreactive and have little agricultural value. Quality varies according to the source and subsequent milling – it is good to check the lime quality before you apply it.
Lime is Calcium and Magnesium Carbonate. Lime adds add Calcium and Magnesium to the soil and the carbonate reacts with acids in the soil (like H+) to increase up the soil pH. Lime can be used in acidic soils, the amount required is determined from a complete soil analysis – which takes into account the pH, H+, Ca2+, other cations and soil CEC (heaviness) in a scientific way to balance pH and cations properly.
Applying too much lime is called over-liming and increases the soil pH. At high soil pH many nutrients get locked up and plants start yellowing. The high soil pH can be counteracted with acidic drip water or acidic fertilizers like Ammonium Sulphate. Nitric acid is preferred to bring in the extra Nitrates that are missing from the Calcium Nitrate application.
Sulphuric acid can work and is cheap, but has two downsides. One it is very dangerous to handle and is not allowed by some certificates. Sulphuric acid brings in excess sulphates that may temporarily tie the Calcium up as semi-soluble gypsum. Phosphoric acid can bring in too much phosphates that will tie up Calcium and iron.
2HNO3 + CaCO3 —–> Ca(NO3)2 + H2O + CO2
Calcitic lime reacts with Nitric acid to produce Calcium Nitrate, water and carbon dioxide. Amounts depend on the strength of the acid and the quality of the lime.
Generally, standard limes may not react fast enough to supply enough Calcium. For hydroponics you need a more readily available calcium source. Soil is more buffered and as long as there is enough Calcium in the soil – it is just a matter of regularly replacing plant removals by top dressing.
Granulated micronized limes are more expensive due to the additional processing & they are imported. But they are much easier to measure out and apply & there is less dust. The granules collapse when wet and the micronized lime particles quickly move into root zone and dissolve fast. So, they deliver calcium faster – but they also change the pH faster.
An idea would be to top dress the granules at the calculated rate of weekly calcium application (from your weekly Calcium Nitrate application per ha). For hydroponics you would apply a smaller rate more often and closely monitor with media and drain analysis until you get a balance.
Closely measure pH and counteract the alkalinity with extra acid and or acidic fertilizers. Always take your water quality into account. High bicarbonate water needs to be neutralized. Granulated limes include Calciprill and Magprill from Omya (Lachlan), G Lime from Amiran, Liquid lime from Dudutech.
Gypsum contains Calcium Sulphate. It is available locally and relatively in-expensive. It should be tested as the quality varies from source to source. It is only sparingly soluble in water at a rate of about 2.2 g/liter. It does not affect the pH directly but can cause an increase in EC and sulphates.
Normal gypsum is fine for top dressing soils if you have enough calcium in your soil profile and are just topping up the plant removals. There are also micronized and ‘soluble’ gypsum products that will move into solution much faster and would be more suitable for hydroponics. Ezy Flow Gypsum from Dudutech is a micronized liquid gypsum that can be drenched.

Solid lime and gypsum products should not be applied via drip – they are very insoluble and will block your irrigation system. They should be sprinkled on the surface between the plants and watered in with a showerhead on a hose. The drip water will then pick them up and move them through the profile. Liquid gypsum and lime products should be applied alone as a drench as the last irrigation of the day.
Calcium Ammonium Nitrate (CAN) is not fully soluble and only suitable for top dressing. It is hygroscopic and quickly dissolves in absorbed water from the air, and then can move into the soil/media. Fertilizer grade CAN contains roughly 8% calcium and 21-27% nitrogen.
It is less acidifying than Ammonium Sulphate and brings some Calcium into the equation. It tends to have about 13% nitrate and 13% ammonium ions. It should be used sparingly and only after trials in your system. Ammonium ions compete with Calcium ion uptake and can cause a rapid soft growth flush.
This is a micro-encapsulated liquid calcium that can be drenched and remains available in the soil solution for a long time – manufactured by Cosmocel, available from Ocean. (20% Calcium) (no sulphates, nitrates, carbonates or chlorides).
ICL have a range of fertilizers suitable for topdressing that contain varying levels of calcium and other nutrients. (Agroleaf Power Calcium 11-5-19+9CaO+2.5MgO+TE, Nutrivant calcium 12-5-27+8CaO+TE, Polysulphate – 17%CaO).
ETG Kynoplus Nafaka 18-38-0+5S+2.3Ca+0.2MgO, Kynoplus Horti 15-9-21+4.75S+2.9Ca+1.5MgO.
There are also a number of drenches that can bring in calcium – Dekompakt 9% Ca(Amiran), Trafos Green Plus 15.2% Ca, (Elgon), Codasol Plus 12.5%Ca (Twiga), Barrier 14.8% Ca (Ocean), Osa Calpower 5%Ca, (Novixa).
Apply calcium drenches as the last cycle of the day to get maximum uptake in your plants.
There are also many different foliar sprays to boost calcium levels in the plant. Note that Calcium moves through the xylem – up the plant, very little is moved in the phloem down to the roots. You always need some Calcium in the root zone. Green flower buds and fruit (eg tomatoes) – have less stoma and tend to be at the ends of the stems – concentrate foliar sprays here for better uptake.
Foliar sprays don’t just contain Calcium – look at the other ingredients too. Some foliar sprays are designed for 1-2 sprays in an orchard/coffee plantation per season and contain high boron or zinc and are not suitable for weekly spraying. ALWAYS read the label and do a phytotoxicity test first. (OmyaPro calcium is a 35% Ca pure calcium foliar). Calcium foliar sprays must be applied regularly and concentrate on the new growth.
Always consider the whole picture when it comes to nutrient application – add up the irrigation water / fertigated nutrients and topdressing nutrients and keep a balance.
Complete soil analysis at planting and addition of scientifically calculated lime and gypsum to balance calcium levels in soil. 500 kg calcium nitrate is used in fertigation daily over 20 ha.
The fertigation recipe is adjusted to reduce Calcium nitrate by 50%, using Potassium Nitrate and Magnesium Nitrate to increase the Nitrate levels and lower the Sulphate levels in the fertigation program. The balance of N is added as urea. The N:NH3 ratio in fertigation kept below 20% N:NH3. The EC is reduced. The ppm N in the final drip water remains constant.
250 kg/day /20 ha calcium nitrate reduction = 3500 kg/20 ha/ 14 days. ie 175 kg CaNO3 per ha per 2 weeks (34 kg calcium). This works out as a topdressing of 150kg/ha gypsum (225 Ca) every two weeks, sprinkled on and washed into the soil with showerheads.
Monitor available nutrients with a 1:2 soil analysis every month and leaf nutrients levels with a leaf analysis every two weeks & adjust feeding program until the situation has stabilized.
To source lime / gypsum / calcium fertilizers and foliar feeds please visit http://shambaza.com/ . To adjust recipe’s, test soils / hydroponics or leaves please contact us on support@cropnuts.com
Happy farming!

Ruth Vaughan is the Technical Director at Crop Nutrition Laboratory Services Ltd. (CROPNUTS). Ruth is also a contributing author to Kenya’s leading horticulture magazines such as the HortFresh Journal, HortiNews and Floriculture. Ruth is a great believer in soil health, organic matter, biochar and carbon sequestration as a way to alleviate climate change and increase food security. Loves visiting farmers and seeing all the different farming methods
Order our services and get to know how to improve your soil for better yeilds.