
In Africa, and especially in Kenya, high sodium soil is one of the major causes of yield losses, quality reduction and crop failure in irrigated agriculture. So today, in our first newsletter, we are going to talk about sodium in the soil.
Soils with a high percentage of sodium in the soil are called sodic soils. Sodic soils negatively impact plant growth for several reasons:-
In low rainfall areas with high evaporation, like many parts of Africa, Sodium and other salts build up in the soil surface over time. This effect is rapidly enhanced due to over grazing, which removes the plant cover and compacts the soil, reducing water infiltration and causing a salt build up.
In coastal areas Sodium comes from sea spray and sea salt in the rain. Irrigating the soil with water containing high Sodium is a major cause of high soil sodium in Kenya. It is important to identify the root cause of the sodium build up so you can deal with it. If you are irrigating, the FIRST thing to test is the water quality, with an irrigation water analysis.
High Sodium levels compete with Calcium, Potassium and Magnesium for uptake by plant roots. Some plants take Sodium up with a high affinity (sodium sensitive plants). Sodium sensitive plants include potatoes, beans, woody plants, vines and stone fruits. Sodium toxicity can be seen as necrosis of leaf tips and plant yellowing like these tomato plants in Thika, and onions in Naivasha (Kenya).

Long before you see the classic toxicity symptoms in the plant leaves – you will notice the plants struggling with high pest and disease pressure. Spidermite infestations are very often associated with high Sodium. Death of plants from Fusarium is very common in sodic soils. Sometimes the Sodium uptake affinity is bred out of plants creating Sodium tolerant varieties. To avoid sodium toxicity, plant Sodium-tolerant plant species or Sodium-tolerant varieties. Don’t struggle with Sodium sensitive plants in high Sodium soils – you will never win.
Soil nutrient imbalances, high pH soil and soil structure can all be improved by getting a complete soil analysis. The soil correction recommendations will help reduce the Sodium in the soil, improve the soil structure and address nutrient imbalances. Ideally this should be done before you plant, but you can still do this in long term crops like roses, while the plants are still growing.
The effect of sodium on soil structure depends on the amount of clay or silt in the soil. The cation exchange capacity (CEC) gives an indication of soil type. Heavy soils with a high CEC generally have a high clay content. Fine silty soils, like those in Naivasha, are also highly affected by Sodium. The Sodium attaches to the soil particles making them repel each other. This disperses the soil and breaks down the soil crumb structure. These small particles move in the soil water and block the soil pores that are so important for root growth, gaseous exchange (oxygen in and carbon dioxide out) and water movement.

Sodium levels are reported as Exchangeable Sodium Percentage (ESP) in your complete soil analysis. At an ESP of <5% the soil structure starts to break down, and water infiltration reduces. As water infiltration reduces, the Sodium quickly builds up, creating a rapid upward spiral in Sodium levels and a fast collapse of soil properties and plant health. At an ESP of 15%, pretty much everything goes wrong and the soil is very difficult and very expensive to rehabilitate. The earlier you deal with Sodium the better.

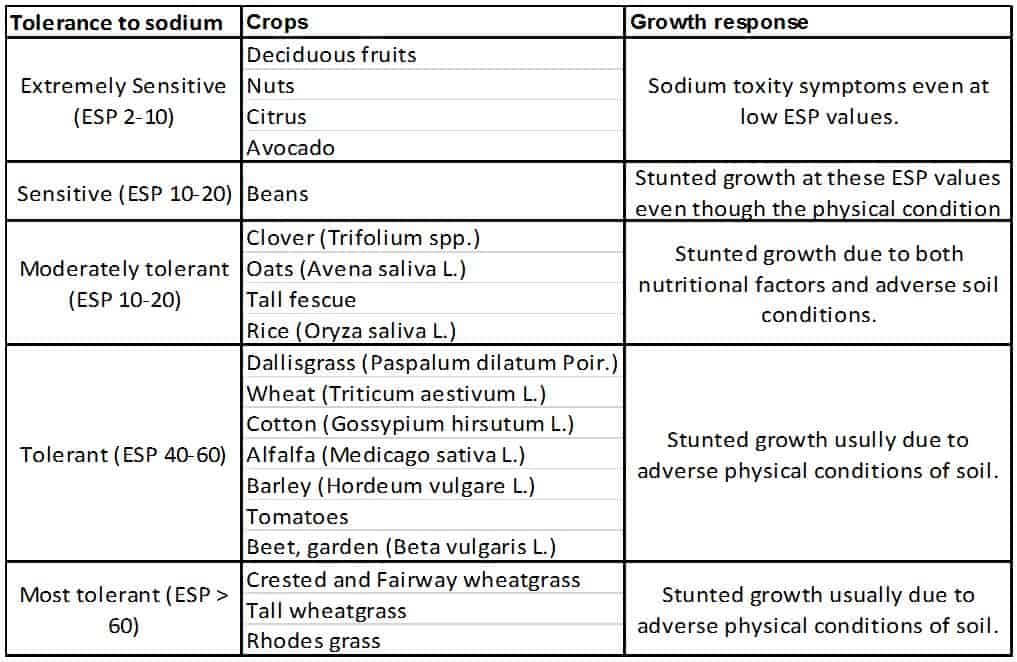
Some plants are very intolerant to Sodium. If you have high Sodium in your soil or water do not embark on the uphill struggle of trying to grow sodium sensitive plants – choose plant species or plant varieties that are sodium tolerant.
Chart of salt tolerance in some common commercial plants
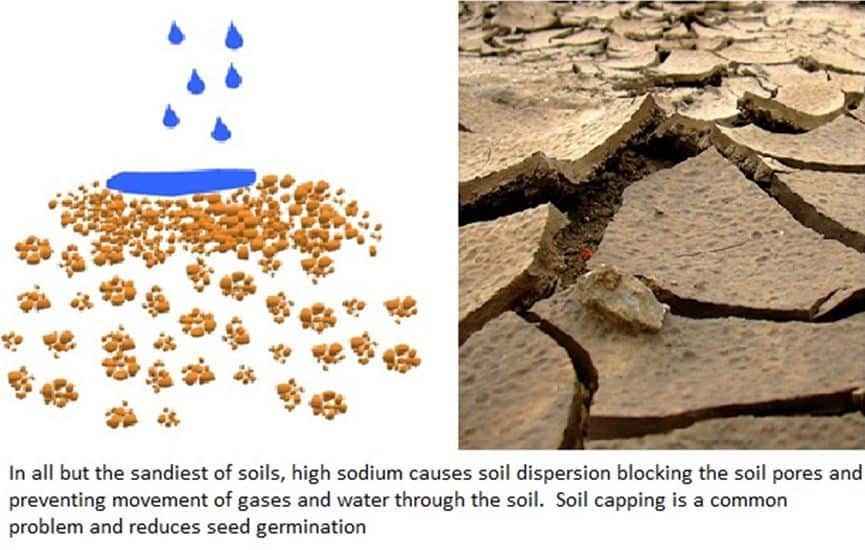
Sodium builds up when water cannot infiltrate easily through the soil. Dig soil pits in your fields to look for a hard pans or concrete layers that prevent water movement and salt leaching. Hard pans are more common in clay based soils, concrete layers in silty soils. These compacted layers need to be physically broken up by deep ripping or deep digging, to shatter, without turning the soil over and burying the valuable topsoil. You cannot remove sodium without leaching which needs free water movement through the soil profile.

High Sodium causes soil dispersion and suspends silt in the soil water solution. This blocks the soil pores and caps the soil which prevents movement of water into and through the soil, causes run off and topsoil loss via erosion and reduces seed germination.
Adding organic matter and humic/fulvic acids will improve the soil crumb structure, increasing the water infiltration rate and leaching potential of the Sodium. Mulching the surface will reduce evaporation and soil capping. Keeping active plant roots and crop residues in the field will further improve soil structure and water infiltration. Building high beds will improve water infiltration and movement of salts away from the root zone. Irrigating with drip irrigation will reduce evaporation and improve water infiltration.

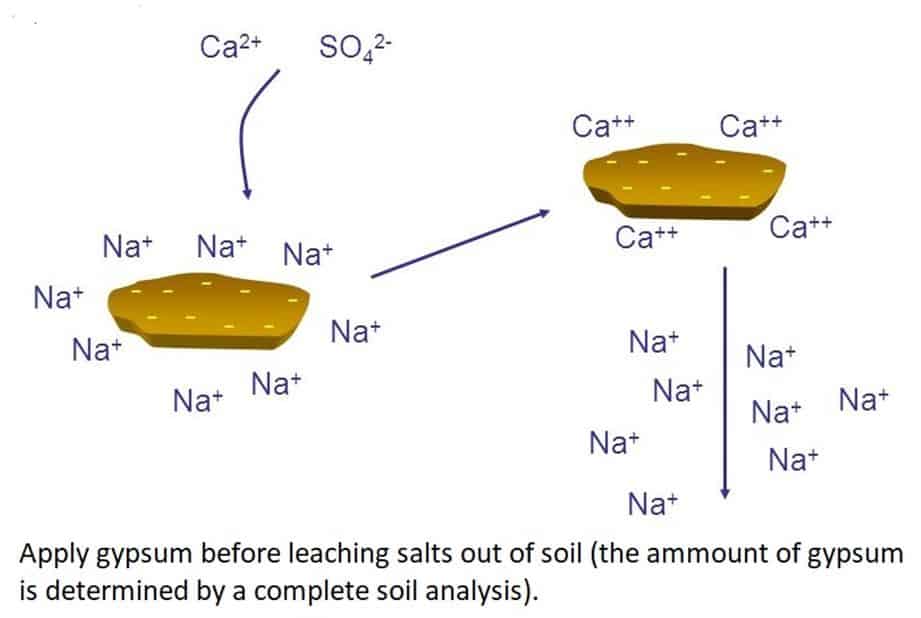
If you are concerned about sodium in your soil, I hope by now that you have had a look at previous soil analysis results or have organized for a proper soil analysis to be done by contacting support@cropnuts.com or your CropNuts Agronomist. Your soil analysis results will have a soil fertility correction recommendation, which tells you the right amount of Gypsum to apply. This is based on correcting the sodium in the top 20 cm of soil.
The Gypsum can be mixed into the soil at planting or surface applied in the case of permanent crops like roses, coffee and fruit trees. If you cannot afford to apply the Gypsum in one go, you can plan the application over a year or two. However the sooner you can apply, the sooner your yields will improve.

How does Gypsum work? The Calcium in the Gypsum has a higher affinity to the negatively charged soil particles than the Sodium and ‘kicks’ the Sodium off into the soil solution where it can be leached out. The Calcium improves the crumb structure of the soil, counteracting the soil dispersion and improving water infiltration, allowing the water with the Sodium to leach out.
Dispersed sodic soils repel water when dry and block up when wet. The EC of the applied water affects its ability to ‘wet’ sodic soil and penetrate the soil structure, fertigation with fertilized water is better than applying fresh water. Keep a fine layer of Gypsum on the soil surface at all times, this improves wettability and brings in extra Calcium to counteract the Sodium. You can also add specialized dispersing agents like humic acids and liquid soaps.
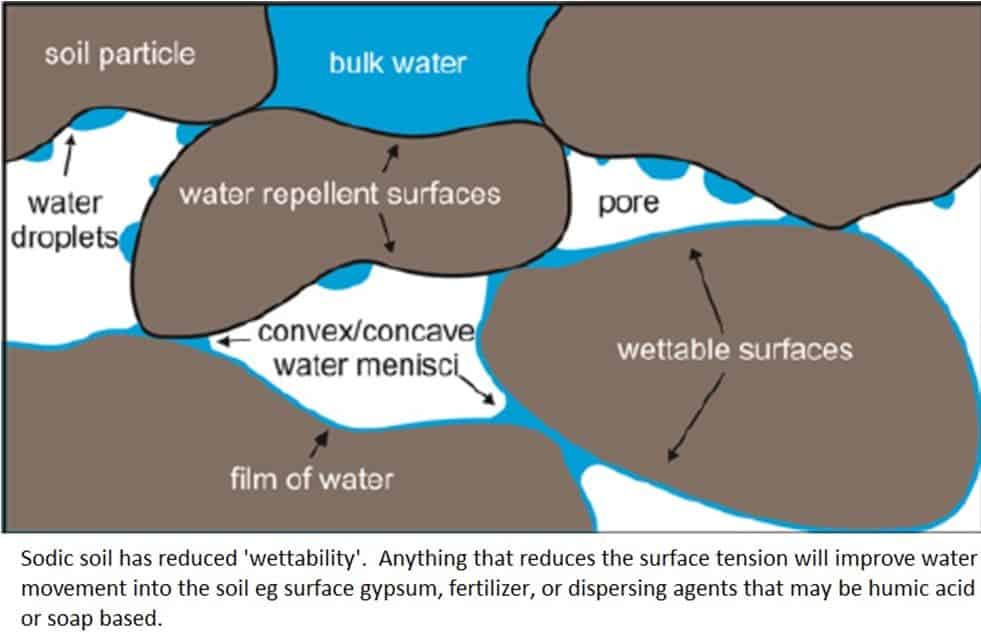
Note that in many volcanic area for example the Rift Valley, very high percentages of potassium can cause similar problems in soil structure.
To look for gypsum or salinity correction products please explore the following links on our on line directory: http://shambaza.com/soil%20compaction
To organize a soil analysis or seek further advice on your existing analysis please contact us on support@cropnuts.com
In the previous sections, we dealt with sodium in the soil and how it can affect plant growth and soil structure. Much of the soil Sodium in irrigated crops comes from bad quality water. If you are planning to irrigate then test the water quality before you start. It’s much easier to deal with Sodium right at the beginning. If you haven’t tested the water I recommend you do so now.
Sodium is a small highly soluble element. All groundwater contains some sodium because most rocks and soils contain Sodium compounds from which the sodium is easily leached. It occurs naturally at elevated levels in many areas of East Africa. The most common sources of high Sodium in ground water are:-
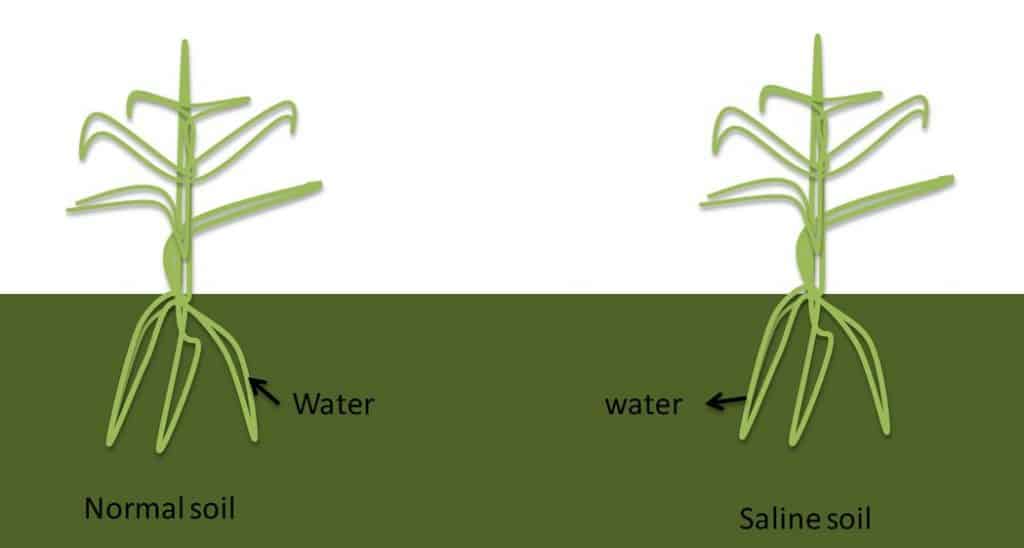
In water, Sodium has no smell but can be tasted by most people at concentration of >200 ppm. Irrigating with poor quality water usually does not have an immediate negative effect on plant growth. Rather the Sodium builds up in the soil and results in a long term hazard eventually reducing soil productivity and killing plants.
Because the salts on the water go through and are deposited in the soil, interpreting water test results is inextricably tied to the soil properties. Sandy soils are much less likely to accumulate sodium than clay or fine textured soils. Leaching is required for Sodium management – soils that are compacted, have fine silts or poor drainage and soils above a high water tables are much more susceptible to sodium accumulation.
Sodium is associated with high salinity water. This is measured as the EC (electrical conductivity) or TDS (total dissolved salts). If saline water is used in irrigation it can create a physiological drought – where the roots struggle to take water up due to the high EC and plants start wilting and die. The salts applied in irrigation are left behind in the soil following evapo-transpiration of the plants, this leads to soil degradation. If sodic or saline water is used; water should be applied generously, over and above the plants’ requirements in order to leach the salts below the root zone and prevent salt build up.
| Total Dissolved Solids – TDS (ppm) | Electrical Conductivity – EC mS cm -1 | Salinity Hazard Irrigation Water |
| 175 | 0-0.25 | Low – acceptable water for all crops in all soils. |
| 175-500 | 0.25-0.75 | Medium – will affect salt sensitive crops – field beans, strawberries, nuts, fruit trees & woody perennials. Leaching required to prevent salt accumulation. |
| 500-1000 | 0.75-2.25 | High – will affect moderately sensitive crops, most grain forage and vegetable crops. Special soil and water management required. Do not use on soils with restricted drainage. Test soils annually. |
| >1500 | >2.25 | Should only be used for salt tolerant crops, eg cotton and barley, in sandy free draining soils, with careful soil and water management. Test soils annually. |
This very important parameter will be found on your irrigation water analysis report. It is calculated from the Sodium, Calcium and Magnesium concentrations in the water. Calcium and Magnesium counteract the negative effect of Sodium in the water and reduce the SAR value. Sodium causes the soil pores to close up and make wetting the soil and water infiltration difficult causing permeability problems.
Low salinity water with high Sodium has lower permeability into soils, than high EC water. Water with a higher EC has less surface tension and will infiltrate the soils more easily in water with a high SAR. The surface tension and infiltration can also be improved by adding wetting agents and humic acids.
To see if the water will have infiltration problems, the SAR and Salinity need to be considered together. You can plot your water quality on the below graph.
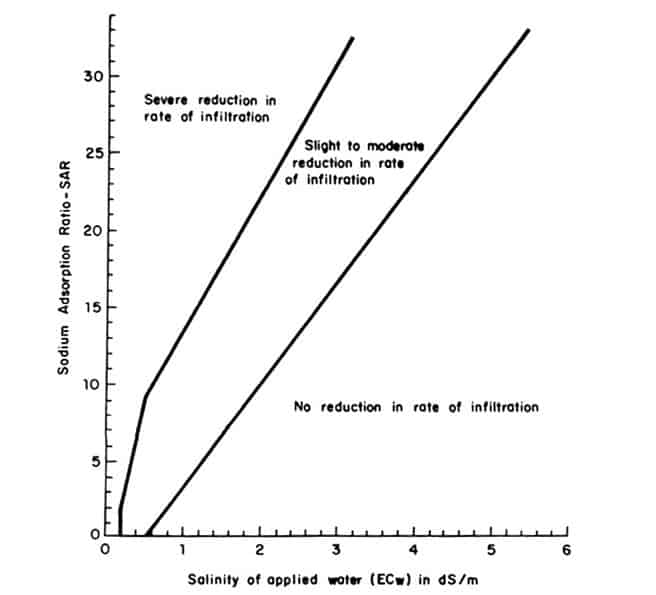
The SAR can be manipulated by adding Calcium and Magnesium to the water. EC can be increased, within reason, by adding fertilizers. Both require a fertigation system. This will allow the use of marginally bad water. Seek professional advice.
Sodium ion also has a toxic ion effect on many plant species. When looking at what crops to grow, always look at the total amount of Sodium in the irrigation water in parts per million. This will guide you on crop choice.
Sodium in water is very often accompanied by chloride ions. Although an essential nutrient to crop growth, toxic levels of chlorine in water can restrict plant growth. Water chloride concentrations up to 70 ppm are safe for all plants. From 70 to 140 ppm chloride, sensitive plants may incur some injury. From 140 to 350 ppm chloride moderately tolerant plants will likely incur injury. Severe problems can be expected at concentrations above 350 ppm chloride. Woody and vine plants and stone fruits are susceptible to chloride toxicity. High sodium and chloride levels in water can also be toxic to leaves when using sprinkler or overhead irrigation water.

These minerals exist as positively charged ions in water, and they counteract the deleterious effect of Sodium. Their concentrations are used in the calculation of SAR. They are plant nutrients and Calcium and Magnesium can be added to the water as part of a fertigation program to reduce the SAR.
Calcium and Magnesium, in conjunction with bicarbonate contribute to the water hardness – and can precipitate out in water, blocking irrigation fittings and pipes. The hardness of the water will guide you on suitable irrigation systems and maintenance treatments.


Potassium is a plant nutrient and has positive effects on plant growth. The ions are very small and mobile and too much potassium can have a similar effect on soils as Sodium. Potassium is generally not found in high levels in water. If it is, you might have problems with Magnesium deficiencies and leaf yellowing in your crop. Most potassium toxicity is man-made.
These are present in water as HCO3- ions. They are alkaline and raise the pH of the water. Measuring your water pH will give an idea that bicarbonate is present but will not give you the level. The only way to find out how much bicarbonate you have in the water is to analyze the water. Bicarbonates react with Calcium and Magnesium in the soil and precipitate out as free lime, raising the soil pH and locking up phosphate and micro-nutrients causing your plants to go yellow.
By precipitating the Calcium and the Magnesium from soil solution, bicarbonates, in the presence of Sodium, can have a very negative impact on your crops and soil structure. Bicarbonates are easily neutralized by adding fertilizer grade acids to your water. These will also add plant nutrients eg nitric acid will add nitrates and phosphoric acid will add phosphates, so seek professional advice to adjust your fertilizer program. Sulphuric acid is generally not recommended for use, because it is caustic and dangerous to handle.
There is no chemical or magic wand that can remove sodium and chloride from the water. If your levels are too high, you should seek professional guidance on a suitable water treatment. This is usually reverse osmosis, which is expensive to install and maintain, so needs to be factored into your budget.

Sodium and free lime can build up in the soil – monitor with complete soil analysis and free lime analysis. Sodium and bicarbonates can have a very disruptive effect in soils with high silt levels – do a soil texture analysis to assess the risk (this is a one off – soil texture should not change).
Surface application of Gypsum will enhance water infiltration of high sodium water and can reduce the effect of the sodium in the water. So can addition of special wetters and organic acids.
Please check these links for products to use.
http://shambaza.com/soil%20compaction
http://shambaza.com/salinity-control
If you suspect you have a sodium problem – send in a soil and water sample for analysis at CropNuts, or have a good look at your existing soil analysis reports or simply talk to us on support@cropnuts.com

Ruth Vaughan is the Technical Director at Crop Nutrition Laboratory Services Ltd. (CROPNUTS). Ruth is also a contributing author to Kenya’s leading horticulture magazines such as the HortFresh Journal, HortiNews and Floriculture. Ruth is a great believer in soil health, organic matter, biochar and carbon sequestration as a way to alleviate climate change and increase food security. Loves visiting farmers and seeing all the different farming methods
Order our services and get to know how to improve your soil for better yeilds.